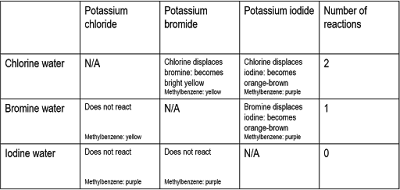
- Nitrogen (78%)
- Oxygen (21%)
- Argon (0.9%)
- Carbon dioxide, Water vapour, etc. (>0.1%)
The last category includes greenhouse gases: Gases that trap the sun's energy inside the atmosphere, causing the greenhouse effect. This is usually a good thing; the greenhouse effect keeps us alive. But excess greenhouse gases are being released into the atmosphere, increasing the volume constantly (due to burning fossil fuels, etc.). This causes the enhanced greenhouse effect, where too much of the sun's energy is trapped and causes a heating effect called climate change. This is a dangerous process that is threatening the world we live in, and must be stopped.
We can work out the percentage volume of oxygen in air with a simple experiment.
- Place a tube containing copper in the middle of two gas syringes (containing a known volume of air), attached so the ends are sealed.
- Gently heat the copper with a bunsen burner, while slowly pressing the syringes, alternating, and keeping an eye on the volume.
- Once the volume stops changing when you press the syringe through, turn off the bunsen burner and wait for it to cool.
- Compare the volume you started with, and the volume you ended up with, and calculate the percentage. This is how much oxygen was in there.
Oxides can be formed by burning elements, for example:
- Burning magnesium forms magnesium oxide, a basic compound
- Burning sulphur forms sulphur dioxide, an acidic compound that can be dissolved to form sulphuric acid
- Burning carbon to form carbon dioxide, an acidic compound which has many uses but also contributes greatly to the enhanced greenhouse effect.
Carbon dioxide is water-soluble, making it useful for carbonating drinks. The carbon dioxide is dissolved under high pressure, but as this is a reversible reaction when the pressure is released bubbles form.
It is also denser than air, making it useful for smothering fires. CO2 is used in many fire extinguishers for this reason.
Carbon dioxide can be formed by reacting hydrochloric acid and calcium carbonate:
Hydrochloric acid + Calcium carbonate --> Carbon dioxide + Calcium chloride + Water
HCl(aq) + CaCO3(s) --> CO2(g) +CaCl2(s) + H2O(l)
Calcium carbonate could be used as marble or limestone, and dropped into a sealed flask of dilute hydrochloric acid in small pieces. A delivery tube could be placed in the end of the bung to allow for the gas to be collected in the downwards displacement method.
Carbon dioxide can also be formed through the thermal decomposition of a metal carbonate, for example:
Copper (II) Carbonate --> Carbon Dioxide + Copper Oxide
CuCO3 --> CO2 + CuO
Another decomposition reaction is hydrogen peroxide heated with manganese (IV) oxide.
Hydrogen peroxide --> Water + Oxygen
It is also denser than air, making it useful for smothering fires. CO2 is used in many fire extinguishers for this reason.
Carbon dioxide can be formed by reacting hydrochloric acid and calcium carbonate:
Hydrochloric acid + Calcium carbonate --> Carbon dioxide + Calcium chloride + Water
HCl(aq) + CaCO3(s) --> CO2(g) +CaCl2(s) + H2O(l)
Calcium carbonate could be used as marble or limestone, and dropped into a sealed flask of dilute hydrochloric acid in small pieces. A delivery tube could be placed in the end of the bung to allow for the gas to be collected in the downwards displacement method.
Carbon dioxide can also be formed through the thermal decomposition of a metal carbonate, for example:
Copper (II) Carbonate --> Carbon Dioxide + Copper Oxide
CuCO3 --> CO2 + CuO
Another decomposition reaction is hydrogen peroxide heated with manganese (IV) oxide.
Hydrogen peroxide --> Water + Oxygen