F - Fluorine - a pale yellow gas
Cl - Chlorine - a green gas
Br - Bromine - an orange liquid
I - Iodine - a purple solid
At - Astatine - a black solid
The halogens are in the same group, so have similar properties that show trends. As you go down the group, the elements get darker in colour, less reactive, and have a higher melting and boiling point.
The reason for this is because as the molecules increase in size, the distance between the valence electrons and the nucleus increases, weakening the forces of attraction and making it more difficult for the atom to attract another electron. Additionally, the increase in molecule size means the attraction between the molecules is more difficult to break, causing the increase in melting and boiling point.
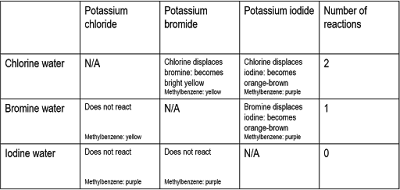
This topic focuses primarily on fluorine, chlorine and bromine. Their reactivity series can be determined by combining a metal halide and aqueous halide, and seeing if a reaction takes place. By adding methylbenzene, we can see which molecules are present (purple is iodine, yellow is bromine)
This shows that chlorine is the most reactive of the three, and iodine the least. These are displacement reactions, where the less reactive halogen is replaced by the more reactive halogen. The more reactive one is reduced, it gains electrons, and the less reactive one is oxidised, it loses electrons.
Reactions






Bromine is a chemical element with the symbol Br and an atomic number 35. It is a fuming red-brown liquid at room temperature. Organic bromine compounds are easily dissociated to generate free bromine atoms at high temperatures, bromine series
ReplyDelete