1.28 describe the formation of ions by the gain or loss of electrons
An ion is formed when an atom loses an electron (It is reduced) or gains an electron (it is oxidised). This change in electron number means the atom has gained a charge. If it has been oxidised, it will become a negative ion: an anion. If it has been reduced, it will become a positive ion: a cation. In ionic bonding, one or more electrons is transferred from one atom to another, turning both into oppositely charged ions which are strongly attracted to one another.
1.29 understand oxidation as the loss of electrons and reduction as the gain of
electrons
OILRIG:
Oxidation
Is
Loss of electrons
Reduction
Is
Gain of electrons
1.30 recall the charges of common ions in this specification
Lithium: Li+
Chlorine: Cl-
Fluorine: F-
Hydrogen: H+
Potassium: K+
Magnesium: Mg2+
Oxygen: O2-
General rule: non-metals are negative, metals are positive
1.31 deduce the charge of an ion from the electronic configuration of the atom
from which the ion is formed
You can look at the outer shell of an atom to determine its ionic charge. If the atom has 2 outer shell electrons, it will lose them and have a charge of 2+. If it has 5 outer shell electrons, it will gain 3, leaving it with a charge of 3-.
1.32 explain, using dot and cross diagrams, the formation of ionic compounds by
electron transfer, limited to combinations of elements from Groups 1, 2, 3
and 5, 6, 7
Ionic bonding is between a metal and a non-metal, the outer shell electrons transferring from the metal to the non metal to give both a full outer shell.
1.33 understand ionic bonding as a strong electrostatic attraction between
oppositely charged ions
The ions become oppositely charged when the electrons are transferred. They experience strong forces of attraction, because opposite charges attract. This forms a strong ionic bond.
1.34 understand that ionic compounds have high melting and boiling points
because of strong electrostatic forces between oppositely charged ions
After transferring electrons, atoms become charged ions which experience strong attraction to opposite charges. This means the electrons are bound by strong electrostatic forces of attraction in this structure:
The strong forces of attraction require a lot of energy to be broken, which is why they have a high melting and boiling point.
1.35 understand the relationship between ionic charge and the melting
point and boiling point of an ionic compound
The bigger the charge, the higher the melting and boiling point as higher charge means stronger forces of attraction.
1.36 describe an ionic crystal as a giant three-dimensional lattice
structure held together by the attraction between oppositely
charged ions
Ionic crystals are held together by the strong forces of attraction between the positively and negatively charged ions. This forms a giant structure, meaning it repeats over and over, and it is a three-dimensional lattice, meaning it is a regular structure that has gaps etc. that allow light to pass through; this is why salt crystals are translucent.
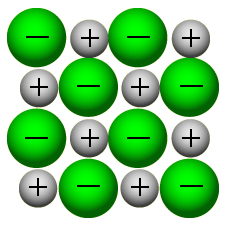
1.37 draw a diagram to represent the positions of the ions in a crystal of
sodium chloride.
Green: Chlorine
White/Grey: Sodium
Subscribe to:
Post Comments (Atom)
Section 3 a) Specification
3.1 explain the terms homologous series, hydrocarbon, saturated, unsaturated, general formula and isomerism. A homologous series is a grou...

-
The group 7 elements, also known as halogens, are F, fluorine, Cl, chlorine, Br, bromine, I, iodine, and At, astatine. They all have antimic...
-
2.29 understand that metals can be arranged in a reactivity series based on the reactions of the metals and their compounds: potassium, sodi...
-
3.1 explain the terms homologous series, hydrocarbon, saturated, unsaturated, general formula and isomerism. A homologous series is a grou...





No comments:
Post a Comment